Liquids Can Be Poured But Solids Cannot. Which Statement Explains This . — the particles in a solid are fixed tightly together. The particles in a solid have little kinetic energy and do not move at all unless energy is supplied. The substance has a volume. — a solid has definite volume and shape, a liquid has a definite volume but no definite shape, and a gas has neither a definite volume nor shape. solids and liquids cannot be easily compressed, but gases can. This means that they cannot move or flow past or over each other and explains why solids cannot be compressed or poured from a container. 9780132525763 matta, staley, waterman, wilbraham. What happens when a solid is dissolved into a. This model explains the properties of. Easily because there is lots of space between the. Terms in this set (10) michael has a substance that he puts in container 1. the three states of matter can be represented by the particle model. the particles move enough that they are not fixed in place, and the liquid can flow.
from studylib.net
The particles in a solid have little kinetic energy and do not move at all unless energy is supplied. — a solid has definite volume and shape, a liquid has a definite volume but no definite shape, and a gas has neither a definite volume nor shape. Easily because there is lots of space between the. This model explains the properties of. What happens when a solid is dissolved into a. the three states of matter can be represented by the particle model. This means that they cannot move or flow past or over each other and explains why solids cannot be compressed or poured from a container. solids and liquids cannot be easily compressed, but gases can. Terms in this set (10) michael has a substance that he puts in container 1. the particles move enough that they are not fixed in place, and the liquid can flow.
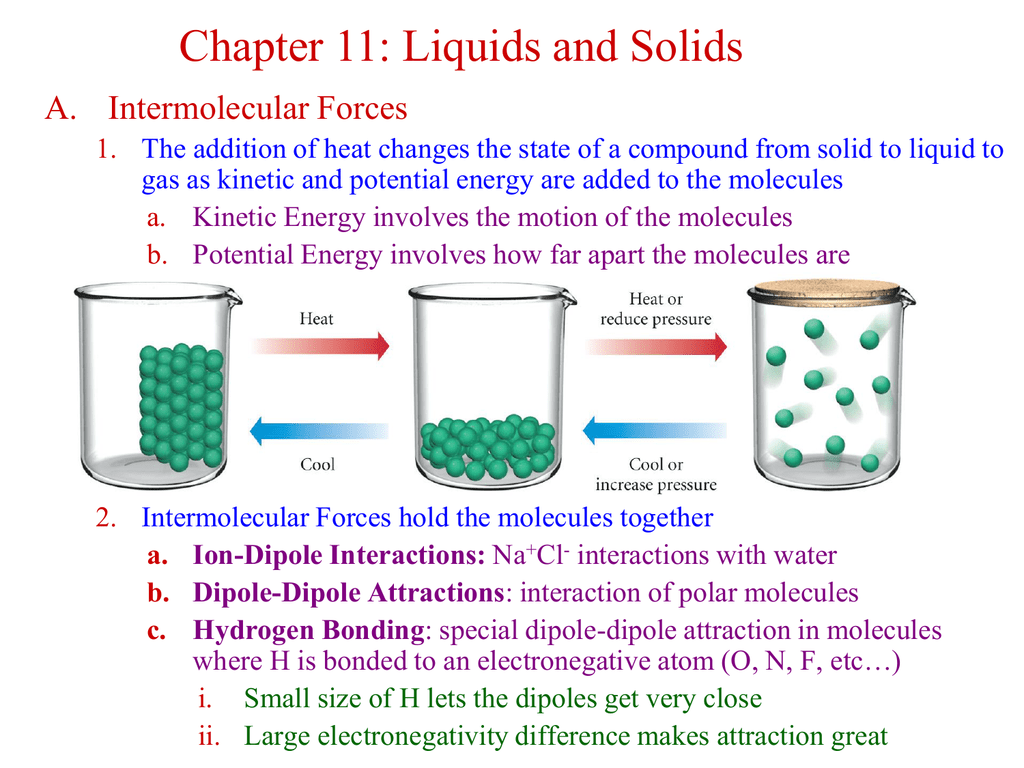
Chapter 11 Liquids and Solids A. Intermolecular Forces
Liquids Can Be Poured But Solids Cannot. Which Statement Explains This This means that they cannot move or flow past or over each other and explains why solids cannot be compressed or poured from a container. This model explains the properties of. What happens when a solid is dissolved into a. Terms in this set (10) michael has a substance that he puts in container 1. This means that they cannot move or flow past or over each other and explains why solids cannot be compressed or poured from a container. the particles move enough that they are not fixed in place, and the liquid can flow. The substance has a volume. The particles in a solid have little kinetic energy and do not move at all unless energy is supplied. the three states of matter can be represented by the particle model. — the particles in a solid are fixed tightly together. Easily because there is lots of space between the. solids and liquids cannot be easily compressed, but gases can. — a solid has definite volume and shape, a liquid has a definite volume but no definite shape, and a gas has neither a definite volume nor shape. 9780132525763 matta, staley, waterman, wilbraham.
From www.exploringnature.org
Phases of Matter Gas, Liquids, Solids Liquids Can Be Poured But Solids Cannot. Which Statement Explains This The substance has a volume. The particles in a solid have little kinetic energy and do not move at all unless energy is supplied. This model explains the properties of. Terms in this set (10) michael has a substance that he puts in container 1. the three states of matter can be represented by the particle model. —. Liquids Can Be Poured But Solids Cannot. Which Statement Explains This.
From www.slideserve.com
PPT SOLIDS LIQUIDS GASES PowerPoint Presentation, free download ID998654 Liquids Can Be Poured But Solids Cannot. Which Statement Explains This 9780132525763 matta, staley, waterman, wilbraham. The substance has a volume. — the particles in a solid are fixed tightly together. the three states of matter can be represented by the particle model. the particles move enough that they are not fixed in place, and the liquid can flow. Easily because there is lots of space between the.. Liquids Can Be Poured But Solids Cannot. Which Statement Explains This.
From thehungryjpeg.com
Different states of matter solid, liquid, gas vector diagram By Microvector TheHungryJPEG Liquids Can Be Poured But Solids Cannot. Which Statement Explains This the three states of matter can be represented by the particle model. 9780132525763 matta, staley, waterman, wilbraham. — the particles in a solid are fixed tightly together. Easily because there is lots of space between the. — a solid has definite volume and shape, a liquid has a definite volume but no definite shape, and a gas. Liquids Can Be Poured But Solids Cannot. Which Statement Explains This.
From dxodcynea.blob.core.windows.net
For Solid Liquid Mixture at Janell Levitt blog Liquids Can Be Poured But Solids Cannot. Which Statement Explains This — the particles in a solid are fixed tightly together. This means that they cannot move or flow past or over each other and explains why solids cannot be compressed or poured from a container. This model explains the properties of. the three states of matter can be represented by the particle model. 9780132525763 matta, staley, waterman, wilbraham.. Liquids Can Be Poured But Solids Cannot. Which Statement Explains This.
From www.thoughtco.com
Liquid Definition in Chemistry Liquids Can Be Poured But Solids Cannot. Which Statement Explains This the particles move enough that they are not fixed in place, and the liquid can flow. Terms in this set (10) michael has a substance that he puts in container 1. The substance has a volume. solids and liquids cannot be easily compressed, but gases can. the three states of matter can be represented by the particle. Liquids Can Be Poured But Solids Cannot. Which Statement Explains This.
From ar.inspiredpencil.com
Liquid Particle Model Liquids Can Be Poured But Solids Cannot. Which Statement Explains This solids and liquids cannot be easily compressed, but gases can. This model explains the properties of. 9780132525763 matta, staley, waterman, wilbraham. — a solid has definite volume and shape, a liquid has a definite volume but no definite shape, and a gas has neither a definite volume nor shape. Terms in this set (10) michael has a substance. Liquids Can Be Poured But Solids Cannot. Which Statement Explains This.
From sciencenotes.org
10 Examples of Solids, Liquids, Gases, and Plasma Liquids Can Be Poured But Solids Cannot. Which Statement Explains This — the particles in a solid are fixed tightly together. solids and liquids cannot be easily compressed, but gases can. This model explains the properties of. The substance has a volume. the particles move enough that they are not fixed in place, and the liquid can flow. 9780132525763 matta, staley, waterman, wilbraham. Terms in this set (10). Liquids Can Be Poured But Solids Cannot. Which Statement Explains This.
From kids.britannica.com
solid Students Britannica Kids Homework Help Liquids Can Be Poured But Solids Cannot. Which Statement Explains This The substance has a volume. What happens when a solid is dissolved into a. The particles in a solid have little kinetic energy and do not move at all unless energy is supplied. 9780132525763 matta, staley, waterman, wilbraham. Terms in this set (10) michael has a substance that he puts in container 1. the three states of matter can. Liquids Can Be Poured But Solids Cannot. Which Statement Explains This.
From guidelistbaquantising.z13.web.core.windows.net
Venn Diagram For Solids Liquids And Gases Liquids Can Be Poured But Solids Cannot. Which Statement Explains This The particles in a solid have little kinetic energy and do not move at all unless energy is supplied. The substance has a volume. This means that they cannot move or flow past or over each other and explains why solids cannot be compressed or poured from a container. solids and liquids cannot be easily compressed, but gases can.. Liquids Can Be Poured But Solids Cannot. Which Statement Explains This.
From chemstuff.co.uk
Particle Model of Solids, Liquids and Gases Chemstuff Liquids Can Be Poured But Solids Cannot. Which Statement Explains This The substance has a volume. — the particles in a solid are fixed tightly together. Terms in this set (10) michael has a substance that he puts in container 1. The particles in a solid have little kinetic energy and do not move at all unless energy is supplied. Easily because there is lots of space between the. . Liquids Can Be Poured But Solids Cannot. Which Statement Explains This.
From www.youtube.com
Science Lesson 4 Mixing Solids into Liquids YouTube Liquids Can Be Poured But Solids Cannot. Which Statement Explains This the particles move enough that they are not fixed in place, and the liquid can flow. — the particles in a solid are fixed tightly together. Terms in this set (10) michael has a substance that he puts in container 1. the three states of matter can be represented by the particle model. The substance has a. Liquids Can Be Poured But Solids Cannot. Which Statement Explains This.
From blog-0734275gh.blogspot.com
28+ nett Bild Definition Of Solid Matter Define Matter In Science States Of Matter Physical Liquids Can Be Poured But Solids Cannot. Which Statement Explains This solids and liquids cannot be easily compressed, but gases can. The particles in a solid have little kinetic energy and do not move at all unless energy is supplied. The substance has a volume. the three states of matter can be represented by the particle model. — the particles in a solid are fixed tightly together. This. Liquids Can Be Poured But Solids Cannot. Which Statement Explains This.
From www.visionlearning.com
Properties of Liquids Chemistry Visionlearning Liquids Can Be Poured But Solids Cannot. Which Statement Explains This The substance has a volume. This model explains the properties of. — a solid has definite volume and shape, a liquid has a definite volume but no definite shape, and a gas has neither a definite volume nor shape. What happens when a solid is dissolved into a. the three states of matter can be represented by the. Liquids Can Be Poured But Solids Cannot. Which Statement Explains This.
From www.animalia-life.club
Examples Of Solids Liquids And Gases Liquids Can Be Poured But Solids Cannot. Which Statement Explains This Easily because there is lots of space between the. The particles in a solid have little kinetic energy and do not move at all unless energy is supplied. — the particles in a solid are fixed tightly together. Terms in this set (10) michael has a substance that he puts in container 1. — a solid has definite. Liquids Can Be Poured But Solids Cannot. Which Statement Explains This.
From socratic.org
What are examples of gases, liquids, and solids? Socratic Liquids Can Be Poured But Solids Cannot. Which Statement Explains This The substance has a volume. This model explains the properties of. Terms in this set (10) michael has a substance that he puts in container 1. The particles in a solid have little kinetic energy and do not move at all unless energy is supplied. solids and liquids cannot be easily compressed, but gases can. the three states. Liquids Can Be Poured But Solids Cannot. Which Statement Explains This.
From igcsechemistryrevision.weebly.com
1.1 Understand the arrangement, movement and energy of particles in each of the three states of Liquids Can Be Poured But Solids Cannot. Which Statement Explains This What happens when a solid is dissolved into a. Terms in this set (10) michael has a substance that he puts in container 1. 9780132525763 matta, staley, waterman, wilbraham. The particles in a solid have little kinetic energy and do not move at all unless energy is supplied. the three states of matter can be represented by the particle. Liquids Can Be Poured But Solids Cannot. Which Statement Explains This.
From www.teachoo.com
Difference between Solid, Liquid, Gas in Table Form Teachoo Liquids Can Be Poured But Solids Cannot. Which Statement Explains This the particles move enough that they are not fixed in place, and the liquid can flow. Terms in this set (10) michael has a substance that he puts in container 1. This means that they cannot move or flow past or over each other and explains why solids cannot be compressed or poured from a container. The substance has. Liquids Can Be Poured But Solids Cannot. Which Statement Explains This.
From www.animalia-life.club
Examples Of Solids Liquids And Gases Liquids Can Be Poured But Solids Cannot. Which Statement Explains This solids and liquids cannot be easily compressed, but gases can. — the particles in a solid are fixed tightly together. What happens when a solid is dissolved into a. 9780132525763 matta, staley, waterman, wilbraham. The substance has a volume. The particles in a solid have little kinetic energy and do not move at all unless energy is supplied.. Liquids Can Be Poured But Solids Cannot. Which Statement Explains This.